Developing next-generation cellular engineering technologies for science and medicine
Reprograming cell function
Our research aims to develop new technologies to modify genome sequences, epigenomic regulation, and cellular gene networks in a precise and targeted manner.
Technology and discovery
We apply technologies to correct genetic diseases, direct cell differentiation, promote tissue regeneration, discover novel drug targets, and answer fundamental biological questions regarding gene regulation and genome structure and function.
Our research
Traditionally, biomedical engineers have been limited to mimicking natural biological processes. However, we currently find ourselves at the apex of a Genomic Revolution that has decoded many of the fundamental principles of biological systems. Armed with this new knowledge, we now envision the engineering of a new biology to fulfill previously intractable design criteria for a myriad of solutions to challenges in medicine and science. This cellular and molecular engineering of new biological systems is the overarching theme of our research and teaching interests. Our central objective is to transform these nascent concepts into technologies that lead to tangible benefits to society. These interests are interdisciplinary by nature and include collaborations with researchers in engineering, molecular and cell biology, biochemistry, bioinformatics, cancer biology, computer science, genetics, genomics, neurology/neurobiology, orthopaedics, pediatrics, pharmacology, and surgery.
More specifically, our scientific interests lie in developing innovative methods in molecular and genetic engineering for applications in regenerative medicine, treating genetic disease, and enhancing our understanding of fundamental biological processes. Our work in these areas capitalizes on the products of the Genomic Revolution and modern advances in the fields of genetic reprogramming, gene delivery, protein engineering, stem cell transplantation, and synthetic biology to create biologic approaches with the potential to improve human health. These studies also facilitate a better understanding of complex processes, including organogenesis, cell lineage determination, and gene regulation, that we hope will ultimately lead to improved design of drugs and biotherapeutics. These efforts are largely focused on coordinating changes in cellular processes at the genetic level in order to achieve more precise, robust, and efficient control of cell behavior. This includes manipulating gene expression networks to control cell differentiation or lineage commitment, creating targeted changes to genome sequences to treat genetic disease, and controlling gene delivery and regulation to enhance efficacy and safety for gene therapy. Our unique approach stems from expertise in enabling technologies such as directed molecular evolution, optogenetics, and the rational design of synthetic programmable DNA-targeting systems. Specific biomedical applications of this work include the treatment of genetic diseases such as Duchenne Muscular Dystrophy and the regeneration of diseased or damaged tissues. These intellectual interests are complemented by our commitment to education and outreach at the high school, undergraduate, graduate, and postdoctoral level.
Epigenome editing and gene regulation
Capitalizing on the power of the CRISPR technologies to target epigenetic modifications to specific sites in the genome.
Genome engineering for gene and cell therapy
Developing precise genome targeting technologies to correct mutations that cause genetic disease, induce tissue regeneration by stem cells, or enhance the immune system to treat cancer.
Genetic control systems
Engineering new technologies to maximize our control over kinetics, magnitude, complexity, and diversity of genomic elements.
Programming cell biology
Decoding fundamental principles of cell lineage specification to generate cell sources that would be useful for disease modeling, drug screening, and regenerative medicine.
Genome engineering for gene and cell therapy
Monogenic hereditary diseases are often caused by a single mutation to the more than three billion base pairs of DNA sequence that constitutes the human genome. For the last three decades, a fundamental limitation to the field of gene therapy has been the inability to specifically correct these mutations. As a result, gene therapies to date have almost exclusively focused on methods that insert extra genetic material into random locations within a cell’s genome. The inability to make targeted modifications to cellular genomes has resulted in a myriad of unforeseen negative consequences in experimental and clinical trials of gene therapies. To address these limitations, we are engineering synthetic enzymes that can be programmed to target any location in the genome and catalyze the addition, removal, or exchange of specific gene sequences. Our work falls within the larger field of genome editing for gene and cell therapy that can be applied to diverse diseases and disorders.
A primary example of our work in this area is developing genome editing methods such as zinc finger nucleases, TALENs, and CRISPR/Cas9 to correct mutations to the dystrophin gene that cause Duchenne muscular dystrophy, one of the most common fatal genetic diseases. We have used genome editing to correct mutations in patient cells and demonstrated restored dystrophin expression in cell culture and after transplantation into skeletal muscle in mouse models (Ousterout et al., Molecular Therapy 2013; Molecular Therapy 2015; Nature Communications 2015). More recently, we have extended this work to correcting dystrophin mutations in vivo in mouse models of Duchenne (Nelson et al., Science 2016; Nelson et al., Nature Medicine 2019; Kwon et al., Mol Ther Meth Clin Dev 2020; Pickar-Oliver et al., Molecular Therapy 2021).
Figure 1. CRISPR-based genome editing restores dystrophin expression in mouse models of Duchenne muscular dystrophy. Cross-sections of muscle tissue where the dystrophin protein has been labeled green, including normal, healthy tissue (left), tissue from a mouse model of Duchenne muscular dystrophy (middle), and tissue from the same mouse model that has been treated with the CRISPR gene editing system (right). Nelson et al., Science (2016).
Related work:
Pickar-Oliver A, Gough V, Bohning JD, Liu S, Robinson-Hamm JN, Daniels H, Majoros WH, Devlin G, Asokan A, Gersbach CA. Full-length dystrophin restoration via targeted exon integration by AAV-CRISPR in a humanized mouse model of Duchenne muscular dystrophy. Molecular Therapy 29(11):3243-3257 (2021).
Kwon JB, Ettyreddy AR, Vankara A, Bohning JD, Delvin G, Hauschka SD, Asokan A, and Gersbach CA. In vivo gene editing of muscle stem cells with adeno-associated viral vectors in a mouse model of Duchenne muscular dystrophy. Molecular Therapy Methods and Clinical Development 19:320-329 (2020).
Nelson CE, Wu Y, Gemberling MP, Oliver ML, Waller MA, Bohning JD, Robinson-Hamm JN, Bulaklak K, Castellanos Rivera RM, Collier JH, Asokan A, and Gersbach CA. Long-term Evaluation of AAV-CRISPR Genome Editing for Duchenne Muscular Dystrophy. Nature Medicine 25(3):427-432 (2019).
- Highlighted by NIH Director’s Blog, Duke Today, FierceBiotech, Endpoints News, Science Daily
Nelson CE, Robinson-Hamm, JN, Gersbach CA. Genome engineering: a new approach to gene therapy for neuromuscular disorders. Nature Reviews Neurology 13(11):647-661 (2017).
Nelson CE, Gersbach CA. Engineering Delivery Vehicles for Genome Editing. Annual Review of Chemical and Biomolecular Engineering. 7:637-62 (2016).
ML Maeder and CA Gersbach. Genome Editing Technologies for Gene and Cell Therapy. Molecular Therapy 24(3):430-46 (2016).
CE Nelson, CH Hakim, DG Ousterout, PI Thakore, EA Moreb, RM Castellanos Rivera, S Madhavan, X Pan, FA Ran, WX Yan, A Asokan, F Zhang, D Duan, CA Gersbach. In Vivo Genome Editing Improves Muscle Function in a Mouse Model of Duchenne Muscular Dystrophy. Science 351(6271):403-7 (2016).
- Highlighted by New York Times (Jan 1, 2016, A13), Nature 529:130 (2016), Science Magazine, New England Journal of Medicine 374(17):1686 (2016), BMJ 351 (2016), Nature Reviews Genetics, Nature Reviews Neurology, Nature Reviews Drug Discovery 15(3):160 (2016), Molecular Therapy 24:414-416 (2016), NIH Research Matters, The Guardian, BBC, Stat News, GEN News, and Rare Disease Report.DG Ousterout, AM Kabadi, PI Thakore, WH Majoros, TE Reddy, and CA Gersbach. Multiplex CRISPR/Cas9-Based Genome Editing for Correction of Dystrophin Mutations that Cause Duchenne Muscular Dystrophy. Nature Communications 6:6244 (2015).
- Highlighted by Nature Reviews Neurology 11, 184 (2015), FierceBiotech, and the Muscular Dystrophy AssociationDG Ousterout, AM Kabadi, PI Thakore, P Perez-Pinera, MT Brown, WH Majoros, TE Reddy, CA Gersbach. Correction of Dystrophin Expression in Cells from Duchenne Muscular Dystrophy Patients through Genomic Excision of Exon 51 by Zinc Finger Nucleases. Molecular Therapy 23(3):523-32 (2015).
DG Ousterout, P Perez-Pinera, PI Thakore, AM Kabadi, MT Brown, X Qin, O Fredrigo, V Mouly, JP Tremblay, CA Gersbach. Reading Frame Correction by Targeted Genome Editing Restores Dystrophin Expression in Cells from Duchenne Muscular Dystrophy Patients. Molecular Therapy 21(9):1718-26 (2013).
- Highlighted by Genetic Engineering and Biotechnology News, Muscular Dystrophy Association, and Biotechniques.
Epigenome editing and gene regulation
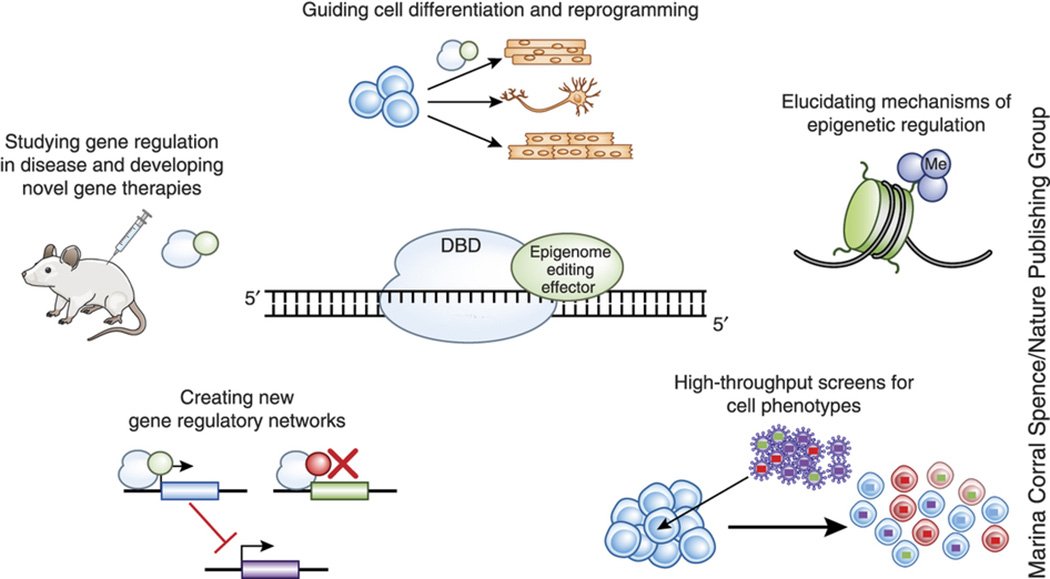
Although our genome sequence provides the instructions that encode for cell functions, the epigenome – or how the genome is structured, modified, and controlled – determines when and to what level those instructions are implemented. Therefore the epigenome is responsible for determining cell type specification, adaptation to environmental stimuli, and response to drugs and other therapies. The epigenome is also misregulated in many diseases and disorders. Interesting, the epigenome plays a central role in biology, disease, and medicine, but is also very poorly understood. Therefore we are developing epigenome editing technologies to better understand epigenetic regulation and harness its power for cell programming (Thakore et al., Nature Methods 2016). A major emphasis of this work is engineering synthetic enzymes that can be used to precisely manipulate any property of the epigenome, including histone modifications and DNA methylation, at very specific locations within the genomic DNA. We are also using these tools to understand the genetics of common complex disease and the determinants of cell fate decisions. Finally, we are interested in the therapeutic applications of these tools to diverse conditions. This work is currently largely focused on capitalizing on the power of the CRISPR/Cas9 system to target these epigenetic modifications to specific sites in the genome.
Figure 1 | Applications of epigenome editing. Targeted control over epigenetic regulation is achieved via fusion of programmable DNA-binding domains (DBDs) to epigenome editing effectors. These engineered epigenome editing proteins can be used for basic research, biotechnology and therapeutic applications.
Related work:
Gemberling M, Siklenka K, Rodriguez E, Tonn-Eisinger KR, Barrera A, Liu F, Kantor A, Cigliola V, Hazlett M, Bartelt L, Bodle J, Rouse DC, Hilton IB, Asokan A, Ciofani M, Poss KD, Reddy TE, West AE, and Gersbach CA. Transgenic mice for in vivo epigenome editing with CRISPR-based systems. Nature Methods 18(8):965-974 (2021).
Pickar-Oliver AK, Black JB, Lewis MM, Mutchnick KJ, Klann TS, Gilcrest KA, Sitton MJ, Nelson CE, Barrera A, Bartelt LC, Reddy TE, Beisel CL, Barrangou R, and Gersbach CA. Targeted Transcriptional Modulation with Type I CRISPR-Cas Systems. Nature Biotechnology 37(12):1493 (2019).
Holtzman L, Gersbach CA. Editing the Epigenome: Reshaping the Genomic Landscape. Annu Rev Genomics Hum Genet. 19:43-71 (2018).
Thakore PI, Kwon JB, Nelson CE, Rouse DC, Gemberling MP, Oliver MO, Gersbach CA. RNA-Guided Transcriptional Silencing In Vivo with S. aureus CRISPR-Cas9 Repressors. Nature Communications 9(1):1674 (2018).
- Highlighted by The Scientist (Jan 1, 2019), Duke Today, Duke Chronicle
Klann TS, Black JB, Gersbach CA. CRISPR-based methods for high-throughput annotation of regulatory DNA. Curr Opin Biotechnol. 52:32-41 (2018).
Klann TS, Black JB, Chellappan M, Safi A, Song L, Hilton IB, Crawford GE*, Reddy TE*, Gersbach CA*. CRISPR-Cas9 epigenome editing enables high-throughput screening for functional regulatory elements in the human genome. Nature Biotechnology. 35(6):561-568 (2017).
- Highlighted by Nature Medicine 23(8):900 (2017), Journal of the American Medical Association 318(5):412 (2017), The Scientist (Jan 1, 2019)
PI Thakore, JB Black, IB Hilton, CA Gersbach. Editing the Epigenome: Technologies for Programmable Transcription and Epigenetic Modulation. Nature Methods 13(2):127-37 (2016).
PI Thakore, AM D’Ippolito, L Song, A Safi, NK Shivakumar, AM Kabadi, TE Reddy, GE Crawford, and CA Gersbach. Highly Specific Epigenome Editing by CRISPR/Cas9 Repressors for Silencing of Distal Regulatory Elements. Nature Methods 12, 1143-1149 (2015).
IB Hilton and CA Gersbach. Enabling Functional Genomics with Genome Engineering. Genome Research 25(10):1442-55 (2015).
LR Polstein, P Perez-Pinera, DD Kocak, CM Vockley, P Bledsoe, L Song, A Safi, GE Crawford*, TE Reddy*, and CA Gersbach*. Genome-Wide Specificity of DNA-Binding, Gene Regulation, and Chromatin Remodeling by TALE- and CRISPR/Cas9-Based Transcriptional Activators. Genome Research 25(8):1158-69 (2015).
IB Hilton, AM D’Ippolito, CM Vockley, PI Thakore, GE Crawford, TE Reddy*, and CA Gersbach*. Epigenome editing by a CRISPR/Cas9-based acetyltransferase activates genes from promoters and enhancers. Nature Biotechnology 33(5):510-7 (2015).
-Highlighted by Nature 528:S12 (2015), Nature 520:135 (2015), Nature Biotechnology 33:606–7 (2015), Nature Methods 12:489 (2015), Nature Reviews Molecular Cell Biology 16:266–7 (2015), Molecular Therapy 23:795 (2015), Genome Medicine 7:59 (2015).
P Perez-Pinera, DD Kocak, CM Vockley, AF Adler, AM Kabadi, LR Polstein, PI Thakore, KA Glass, DG Ousterout, KW Leong, F Guilak, GE Crawford, TE Reddy, CA Gersbach. RNA-Guided Gene Activation by CRISPR-Cas9-Based Transcription Factors. Nature Methods 10(10):973-6 (2013).
-Highlighted by Science 341(6148):833-836 (2013), Molecular Therapy 21(9):1643 (2013), Genetic Engineering and Biotechnology News
P Perez-Pinera, DG Ousterout, JM Brunger, AM Farin, KA Glass, F Guilak, GE Crawford, AJ Hartemink, CA Gersbach. Synergistic and Tunable Human Gene Activation by Combinations of Engineered Transcription Factors. Nature Methods 10(3):239-42 (2013).
-Highlighted by Nature Methods 10(3):207-8 (2013)
Genetic control systems
Much of our work is focused on mimicking, recapitulating, or programming biological systems such as cell differentiation and tissue development. Importantly, in nature these processes are tightly controlled with respect to both space and time. In order to synthetically recapitulate this spatiotemporal patterning, we are engineering systems for the regulation of gene expression with visible light and pharmacologic compounds. This will enable precise spatial and dynamic control of gene expression for modeling the role of gene regulation in development and disease. This work will lead to technologies for studying cell-cell interactions and tissue development, and can also be incorporated into applications such as tissue engineering to create complex features and combinations of tissue types.
Related work:
Kocak DD, Josephs EA, Bhandarkar V, Adkar SS, Kwon JB, Gersbach CA. Engineered Guide RNA Secondary Structure Increases the Specificity of Diverse CRISPR Systems. Nature Biotechnology 37(6):657-666 (2019).
- Highlighted by FierceBiotech, NSF News, and Duke Today
Polstein LR, Juhas M, Hanna G, Bursac N, Gersbach CA. An Engineered Optogenetic Switch for Spatiotemporal Control of Gene Expression, Cell Differentiation, and Tissue Morphogenesis. ACS Synthetic Biology. 6(11):2003-2013 (2017).
LR Polstein and CA Gersbach. Spatiotemporal Control of Endogenous Gene Activation Using Light-Inducible CRISPR-Cas9 Transcription Factors. Nature Chemical Biology 11(3):198-200 (2015).
- Highlighted by BiotechniquesKA Glass, J Link, JM Brunger, FT Moutos, CA Gersbach,* F Guilak*. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 35(22):5921-31 (2014).
LR Polstein, CA Gersbach. Light-Inducible Spatiotemporal Control of Gene Activation by Customizable Zinc Finger Transcription Factors. Journal of the American Chemical Society 134(40):16480-3 (2012).
- Highlighted by Genetic Engineering and Biotechnology News, COSMOS Magazine, Duke’s The Chronicle, and the American Society of Mechanical Engineers (asme.org).CA Gersbach, JM Le Doux, RE Gulderg, AJ Garcia. Inducible Regulation of Runx2-Stimulated Osteogenesis. Gene Therapy 13(11):873-82 (2006).
Programming cell biology
We are interested in developing technologies to precisely program cell biology in order to generate cell sources that would be useful for disease modeling, drug screening, and regenerative medicine. For example, we are pursuing genetic reprogramming as an alternative means for generating various cell sources for science and medicine. This involves reprogramming the gene expression profile of an easily accessible cell type, such as skin cells, by activating the gene network corresponding to a cell type responsible for the regeneration of a specific tissue, such as muscle, bone, cartilage, blood vessels, heart tissue, etc. For example, we are using genetic reprogramming to convert fibroblasts into neuronal cells (Black et al., Cell Stem Cell 2016) and skeletal muscle cells (Kabadi et al., ACS Synthetic Biology 2014). These approaches can be used to better understand the mechanisms of disease and develop optimal patient-specific drug treatments. In the future, these technologies could potentially be used to engineer cells that can regenerate diseased or damaged tissues.
Figure 1. Mouse embryonic fibroblasts converted to neuronal cells by targeted epigenetic remodeling with CRISPR/Cas9-based transcription factors (Black et al., Cell Stem Cell 2016).
Related work:
McCutcheon SR, Swartz AM, Brown MC, Barrera A, McRoberts Amador C, Siklenka K, Humayun L, ter Weele M, Isaacs JM, Reddy TE, Allen AS, Nair SK, Antonia SJ, Gersbach CA. Transcriptional and epigenetic regulators of human CD8+ T cell function identified through orthogonal CRISPR screens. Nature Genetics 55(12):2211-2223 (2023).
15. Black JB, McCutcheon SR, Dube S, Barrera A, Rice GA, Klann TS, Adkar SS, Soderling SH, Reddy TE, and Gersbach CA. Master Regulators and Cofactors of Human Neuronal Cell Fate Specification Identified by CRISPR Gene Activation Screens. Cell Reports 33(9):108460 (2020).
- Highlighted by Genetic Engineering News
Kwon JB, Vankara A, Ettyreddy AR, Bohning JD, Gersbach CA. Myogenic Progenitor Cell Lineage Specification by CRISPR/Cas9-based Transcriptional Activators. Stem Cell Reports 14(5):755-769 (2020).
Black JB, Gersbach CA. Synthetic transcription factors for cell fate reprogramming. Curr Opin Genet Dev. 52:13-21 (2018).
JB Black, AF Adler, HG Wang, AM D’Ippolito, HA Hutchinson, TE Reddy, GS Pitt, KW Leong, and CA Gersbach. Targeted Epigenetic Remodeling of Endogenous Loci by CRISPR/Cas9-Based Transcriptional Activators Directly Converts Fibroblasts to Neuronal Cells. Cell Stem Cell (2016).
TM Gibson and CA Gersbach. Single-molecule analysis of myocyte differentiation reveals bimodal lineage commitment. Integrative Biology 7, 663 - 671 (2015).
BO Diekman*, PI Thakore*, SK O'Connor, VP Willard, JM Brunger, N Christoforou, KW Leong, CA Gersbach*, F Guilak*. Knockdown of the cell cycle inhibitor p21 enhances cartilage formation by induced pluripotent stem cells. Tissue Engineering ;21(7-8):1261-74 (2014).
AM Kabadi, PI Thakore, CM Vockley, DG Ousterout, TM Gibson, F Guilak, TE Reddy, CA Gersbach. Enhanced MyoD-Induced Transdifferentiation to a Myogenic Lineage by Fusion to a Potent Transactivation Domain. ACS Synthetic Biology 3(10):702-3 (2014).
S Chakraborty, H Ji, AM Kabadi, CA Gersbach, N Christoforou, KW Leong. A CRISPR/Cas9-Based System for Reprogramming Cell Lineage Specification. Stem Cell Reports 3(6):940-7 (2014).
KA Glass, J Link, JM Brunger, FT Moutos, CA Gersbach,* F Guilak*. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 35(22):5921-31 (2014).
JM Brunger, NPT Huynh, CM Guenther, P Perez-Pinera, FT Moutos, CA Gersbach,* F Guilak*. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc. Natl. Acad. Sci. USA. 111(9):E798-806 (2014).
CA Gersbach, JM Le Doux, RE Gulderg, AJ Garcia. Inducible Regulation of Runx2-Stimulated Osteogenesis. Gene Therapy 13(11):873-82 (2006).
CA Gersbach, BA Byers, GK Pavlath, AJ Garcia. Runx2/Cbfa1 Stimulates Transdifferentiation of Primary Skeletal Myoblasts into a Mineralizing Osteoblastic Phenotype. Experimental Cell Research 300(2):406-417 (2004).